21. August 2025 By Dr. Anne Tzschichholz and Jessica Hintzen
What the new EU GMP Annex 11 revision and the new Annex 22 really mean for CSV
The IT world has developed rapidly: developments such as cloud services and artificial intelligence (AI) have long been standard. However, they also increase the risk of cyber attacks, which in turn raises the requirements for IT security and data integrity. At the same time, there is a growing desire for digital validation, away from paper-based systems.
These developments have a direct impact on the compliance of computerised systems in the GMP environment. It was therefore only logical to comprehensively revise the previous version of Annex 11 from 2011. The new version responds to technical realities that were not yet in focus more than a decade ago. Many of the earlier formulations were deliberately kept general, but today this is no longer sufficient to reliably regulate the safe operation of modern systems. Annex 11 therefore introduces much more specific, practical and up-to-date requirements. It is supplemented by the new Annex 22, which specifically addresses the use of AI systems in the GMP environment.
But don't worry: anyone who has followed the best practices of the GAMP5 guideline so far is basically on the right track. The new Annex 11 formalises much of what is already considered an established standard in the GAMP5 environment – only now with greater regulatory binding force.
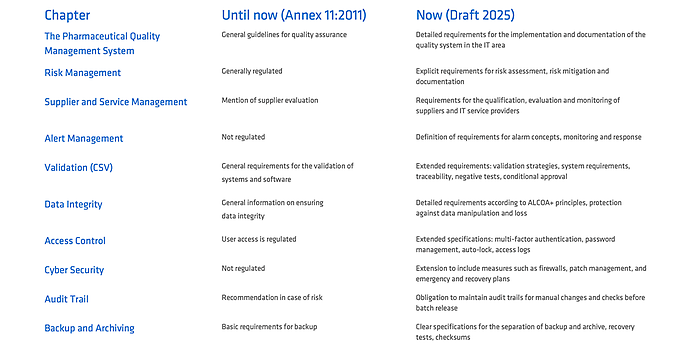
Don't be afraid of the new Annex 11. The most important changes at a glance
We support you!
Do you require assistance in answering the questions or would you prefer an external review to identify your Annex 11 & 22 readiness? Please do not hesitate to contact us.
Four areas of action you should pay particular attention to now
If you have been following the best practices outlined in the GAMP5 guidelines, you are basically on the right track. The new Annex 11 places greater emphasis on certain topics and formalises them. The following are particularly relevant:
Pharmaceutical Quality System (PQS)
The validation of computerised systems is no longer an isolated IT and QM task, but an integral part of the overall PQS. It affects specialist departments, quality assurance and management alike and must be strategically anchored in the company. Effective risk management is essential here: risks must be systematically identified, assessed and minimised through appropriate measures. The focus is on ensuring product quality, patient safety and data integrity throughout the entire life cycle.
Audit trail management
Audit trails must not only be in place, but also regularly reviewed and actively evaluated, especially before quality-related decisions such as batch release.
Data integrity (ALCOA+)
Data integrity requirements are becoming more specific: data must be traceable, unchanged, correct and available throughout the entire life cycle. ALCOA+ principles provide a binding basis for this.
Access controls and cybersecurity
Technical protective measures are mandatory: multi-factor authentication, defined role and rights concepts, patch management, firewalls and a functioning alarm management system must be demonstrably implemented for protection.
And what about AI? Annex 22 introduces additional requirements
Another aspect that is becoming increasingly important is AI in computer-based systems. With the draft of Annex 22, which supplements Annex 11, there is now, for the first time, a separate regulatory framework for AI models in the GMP environment. Annex 22 thus translates the cross-industry EU AI Act (from 2024) into practical requirements for the pharmaceutical GMP context.
The draft makes it clear that the requirements apply whenever AI models are used for prediction, classification or decision support in GMP-critical applications, especially when patient safety, product quality or data integrity are at stake. Annex 22 applies as soon as AI models are integrated into GMP-relevant processes, such as the evaluation of deviations, the classification of raw data or support in release decisions.
Annex 22 also clearly stipulates that AI systems in regulated environments must be transparent, reproducible and validatable. So-called ‘black box’ approaches or self-learning models in productive use are not permitted. Controlled change management is also required: every change to the model, the system or the input data must be documented and evaluated.
Conclusion
With the revision of Annex 11 and the introduction of Annex 22, it is clear that the requirements for computerised systems in the GMP environment are becoming clearer, more specific and more binding. Validation is no longer a mere formality, but a strategic component of the Pharmaceutical Quality System.
Those who have followed the GAMP5 guidelines to date are still well positioned – many of the requirements that are now mandatory were already described there as best practice. Nevertheless, the new requirements raise the bar and close previous loopholes for interpretation.
Now is therefore the right time to subject existing systems, validation documentation and processes to a systematic GAP analysis. The following points in particular should be critically reviewed:
- Is the integration of validation into the PQS documented in a comprehensible manner?
- Are audit trails checked regularly – for example, before batch release?
- Does data management fully comply with the ALCOA+ principles?
- Are cybersecurity, alarm management and access controls implemented in accordance with the current state of the art?
- Are there any AI applications that fall under Annex 22 – and if so, are their risks, decision-making logic and validation adequately described?
Only those who can answer these questions clearly will be able to confidently face future inspections and increasing regulatory requirements.
We support you!
Do you require assistance in answering the questions or would you prefer an external review to identify your Annex 11 & 22 readiness? Please do not hesitate to contact us.


