12. January 2023 By Emily Hossfeld
New support opportunities for people in need of care and their relatives – What is important when developing digital care applications (DiPAs)?
What is a DiPA? Isn’t it called DiGA?
Not exactly – in addition to ‘Digitalen Gesundheitsanwendungen‘ (DiGAs = digital health applications) there will soon be ‘Digitale Pflegeanwendungen‘ (DiPAs = digital care applications). Only on December 02, 2022, the Federal Institute for Pharmaceuticals and Medical Products (BfArM) published the first version of the DiPA guideline describing requirements and application procedure of DiPAs in more detail.
Back in June 2021, DiPAs have been established in social care insurance through the law ‘Digitale-Versorgung-und-Pflege-Modernisierungs-Gesetz’ (DVPMG). As a result, around 4 million people in need of care who are receiving care at home will be entitled to refunds of DiPAs up to 50€ per month. The nursing care fund decides if the person in need of care gets a refund, dependent on their need.
But what is the benefit of DiPAs for people in need of care?
DiPAs reduce impairments in independence and abilities or prevent aggravation of need of care. It needs to be highlighted that DiPAs support in home care exclusively. Those applications may be used by the person in need of care themselves or in interaction with caregivers and may help stabilize the home care situation of the person in need of care.
And how does a digital application become a DiPA?
To be included in the new DiPA directory and thus become a ‘Digitale Pflegeanwendung’ (digital care applications), manufacturers must proof that they meet the requirements for DiPAs as part of a testing procedure executed by BfArM: After the manufacturer submitted the application, the BfArM has three months (in individual cases six months) for checking if the DiPA fulfills the requirements. If this is the case, the application is included in the directory; if the requirements do not apply, the application is turned down.
But what are those requirements for DiPAs?
Besides the regulatory requirements explained below, the application must show the fundamental characteristics of a DiPA: Those characteristics include nursing benefits proved in a quantitative study and the usage by a person in need of care in home care. In addition to reducing impairments of the people in need of care, the nursing benefits may also consist in reducing or avoiding care risks or in supporting volunteer caregivers, such as caring relatives, to stabilize the caring situation – That means, that volunteer caregivers may be another user target group of DiPAs.
When developing a DiPA, it is important that the application provides nursing benefits for the people in need of care in one of the following areas:
- Mobility
- Cognitive and communicative abilities
- Behavioral and psychological problems
- Self-supply
- Coping and self-reliant handling of requirements and strains concerning diseases or therapies
- Arrangement of everyday life and social contacts
- Housekeeping or
- Stabilization of home care.
A key feature of a DiPA is the digital main function; but it may include hardware, such as sensors, if needed for the app’s purpose. In addition, a DiPA may be combined with supplementary support services (Ergänzende Unterstützungsleistungen = eUL) executed by ambulatory care services, to serve a purpose or to meet individual requirements of assistance or selective instructions. All in all, a person in need of care may get a refund of a maximum of 50€ per month, including expenses for the DiPA and supplementary support services.
The regulatory requirements for DiPAs are based on the principle that only easy to use and save applications can be successful in long-term care. The comprehensive requirements are
- Security and functionality
- Data protection and data integrity
- Interoperability and
- Quality.
DiPAs do not have to be medical devices – but they can be. The difference is that the aspects of security and functionality of medical devices are considered as fulfilled because of the conformity assessment procedure according to the Medical Device Regulation (MDR), while non-medical products must meet certain requirements: The safety criteria for non-medical devices need to be proved according to attachment 1 of the DiPA regulation (DiPAV). The attachment is a questionnaire including yes/no statements that must be marked as applicable to meet the requirements.
Attachment 1 of the DiPA predominantly includes requirements for process design for the manufacturer: A quality management system with risk management and defined product lifecycle processes is claimed. To fulfill requirements of data protection and integrity, manufacturers need a certificate from the Federal Office for Information Security (BSI) from 2024; until then, the requirements in the attachment of the DiGA regulation must be transferred for DiPAs. Manufacturers also need a certificate of the implementation of an Information security management system (ISMS) according to ISO 27001; beside that, they must execute a penetration test to ensure a save system at time of application.
The other quality requirements are defined in attachment 2 of the DiPAV. They include requirements for
- Interoperability
- Robustness
- Consumer protection
- Age-based usability
- Usability and accessibility
- Support of people in need of care and users
- Quality of the nursing content and
- Security of the people in need of care (patient safety).
According to the requirements for interoperability, DiPAs must be able to play out human readable, printable data as well as machine readable, interoperable data. Furthermore, they need an interoperable interface for data exchange with medical devices or wearables. Regarding usability and accessibility, manufacturers should stick to norms; to check that the application is self-explanatory, the manufacturer must provide the BfArM free access to the DiPA when submitting the application. To support users, there must be training material and user questions must be answered within 24 hours.
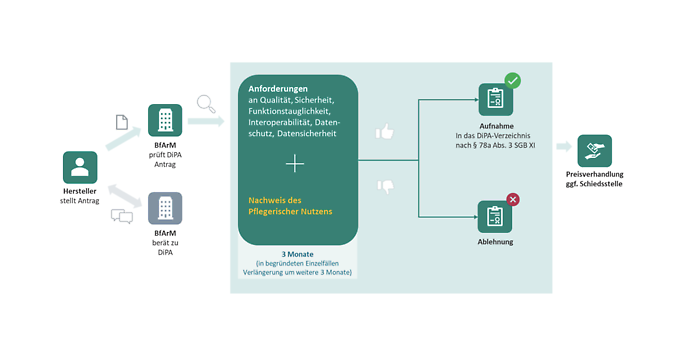
Der Weg von der digitalen Anwendung zur Digitalen Pflegeanwendung (Quelle: DiPA-Leitfaden vom BfArM in der Version 1.0)
Summary: What is important when developing a DiPA?
The picture above (translated from source: DiPA guideline version 1.0) summarizes the path from a digital application to a DiPA: To be recorded in the DiPA directory by BfArM and thus be able to be refunded by the care fund, the focus must be on a native app or web application with a main function offering a proven nursing benefit for people in need of care in a home care context. Furthermore, the manufacturer must prove that the requirements according to security and functionality, data protection and integrity, interoperability, and other quality characteristics, like usability, are fulfilled.
Therefore, it is advisable to procure know-how and information about the requirements your company must meet when developing a DiPA at an early stage. Especially if you do not have any experience in developing and placing medical devices on the market yet, we will be happy to assist you as a qualified partner in the conception, development and business operation of DiPAs.
Further topics and our services for the health sector can be found on our website.
You will find more exciting topics from the adesso world in our latest blog posts..

